Electronic & Radiation Devices
-
-
-
Electronic and Radiation Devices
✤ What is the FDA?In the United States, medical devices are regulated and managed along with foods, pharmaceuticals, and cosmetics under the "Federal Food, Drug & Cosmetic Act: Federal Food, Drug & Cosmetic Act". The safety of the product is secured through pre-inspection and approval of the product, sanctions for nonconforming products through post-inspection, and inspection of imported/exported products.
If you do not comply with the regulations required by the FDA, it cannot be distributed or sold in the United States. Products that do not meet the medical device regulations, even if they are on sale, may be subject to corrective action, recall, product seizure or disposal, criminal disposition, etc. through FDA follow-up management.✤ Related regulations- FD & C Act (Federal Food, Drug & Cosmetic Act)
- 21 CFR (The Code of Federal Regulations)
✤ Coverage- Food, cosmetics, medicines and medical devices (including accessories), veterinary drugs, animal feed, infant formula, food additives, low acid canned food, oxidized food However, in the case of meat, poultry, and processed meat products, inspection and regulations are enforced by the "Food Safety & Inspection Service (FSIS)"
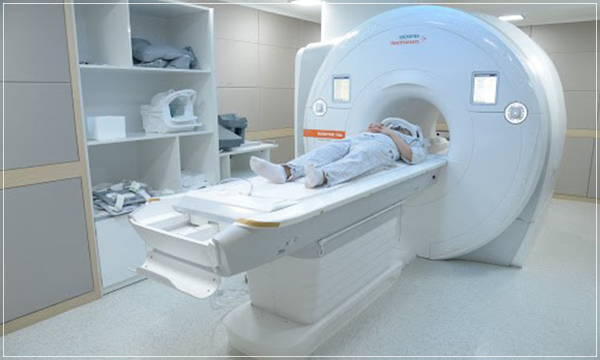
- Radiation equipment (electronic products): Ultrasound treatment equipment, Sunlamp, X-rays, TV receiver, etc.
-
-
-

-
What is Center for Devices and Radiological Health (CRDH)?
- Recommend safe use of radiation by conducting an epidemiological investigation based on the various and potential dangers of radiation.
- Develop measurement methods and tools to evaluate equipment and products that emit radiation.
- Medical devices are classified according to their safety and efficacy (class I, class II, and class III) to be declared and approved before sale.
- By developing working standards for equipment that emit radiation, guidelines for use are prepared to prevent unnecessary exposure to radiation.
- Educate consumers and manufacturers about accurate information and evidence for medical devices and radiation-emitting products.
-
-
-

-
What is accession number?
The accession number is the unique identification number for a report in FDA's database. This number is provided in the CDRH confirmation letter. Market access numbers facilitate communication on specific reports. Because all agencies can use this market access number to determine if a document has been discussed. In addition, FDA import department personnel use accession numbers to ensure that manufacturers have documented requirements for products that are minimally imported.
The accession number not only means that the report has been received into CDRH's filing cabinet, but also means that some of the information about manufacturers and products has been entered into FDA's database.
The confirmation letter will be returned to the address provided by the report submitter. Only one copy will be shipped. If the submitter is different from the manufacturer, the letter will only be sent to the report submitter. Submitters can send a copy to the manufacturer.
Please allow at least 4 weeks after the manufacturer sends the product report, summary report, annual report, or pre-inquiry supplemental report.
If submitted by the manufacturer in electronic report format, an e-mail confirmation message will be sent as soon as the CDRH database has been successfully published. Typically, electronic reports are published in the database within 1 day of receipt.
Manufacturers are required to file a report with CDRH prior to the product being introduced into US commerce. The confirmation letter and accession number are evidence that these reports have been received by the CDRH. If the product was made in another country and then imported into the United States, the import approval process will require the identification of the market access number on the Customs Declaration Form FDA 2877
-
-
-
-
What is electronic product?
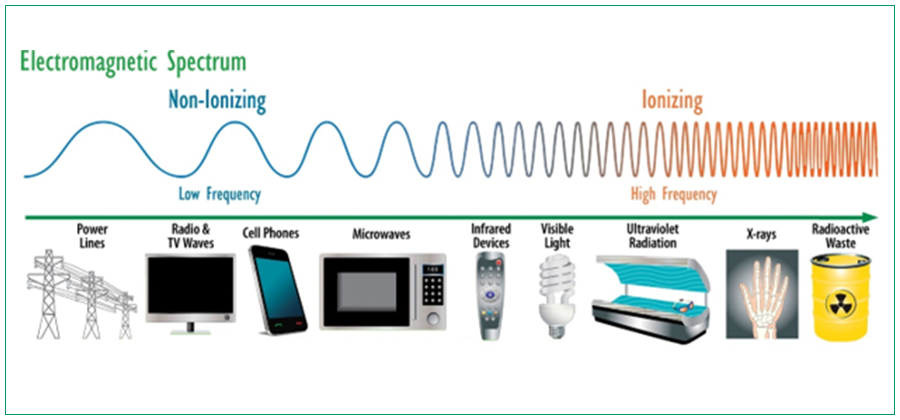
Any product that contains an electronic circuit and generates any kind of radiation is an electronic product that emits radiation. X radiation (x-rays), microwaves, radio waves (radiofrequency (RF)), laser, visible light, sound, ultrasound, and ultraviolet light are a few examples of the many types of radiation-emitting electronic products. Diagnostic x-ray systems, laser products, laser light shows, and microwave ovens are a few examples out of the many different electronic products that emit radiation.
The United States (U.S.) Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) is responsible for regulating radiation-emitting electronic products. The CDRH goal is to protect the public from hazardous and unnecessary exposure to radiation from electronic products. For most electronic products, safety regulation is divided between CDRH and state regulatory agencies. CDRH regulates the manufacture of the products, and the states regulate the use of the products.
Title 21 of the Code of Federal Regulations, Subchapter J, Parts 1000 through 1050 (21 CFR 1000 – 1050) contains radiation safety regulations for manufacturers of radiation-emitting electronic products. Manufacturers are responsible for producing products that do not emit hazardous and unnecessary radiation. All manufacturers must comply with the applicable requirements in Title 21 CFR 1000, 1002, 1003, 1004 and 1005. If a mandatory radiation safety performance standard applies to a manufacturer’s product, then the manufacturer must also comply with Title 21 CFR 1010 and the product must comply with the requirements of the standard. Mandatory radiation safety performance standards are found in 21 CFR 1020 – 1050”
As FDA homepage stated above, FDA requests manufacturer of such products to comply the requirements it they wish to market their products in US.
-
-
-

-
IGC’s Competency
IGC provides differentiated FDA registration consulting services through its US subsidiary Pats corp.
Pats Corp is located in LA, USA, and provides high-quality support for domestic and overseas companies in compliance with US FDA regulations and cGMP.
IGC focuses on preventing problems before they arise in all regulatory requirements that may arise to customers, and provides customers with technical information and best-in-class compliance services. We do our best to support our customers in expanding their business by efficiently and successfully entering the US market.
We will provide accurate, reliable and up-to-date information on the ever-changing FDA legal requirements and provide services in the following categories
-
Related Services from IGC
01System certification
02Product certification (European, Eurasia, U.S., China, Southeast Asia)
03Product testing
04Certification of auditor qualifications
05Professional manpower training and education
