Tel. +82 2 6749 0701
AM 9:00 ~ PM 6:00
Saturday,Sunday,Holiday :
Days Off
In May 2016, the U.S. FDA published its final federal bill, "Food Labeling: Revision of the Nutrition and Supplement Facts Labels," which amended the labeling rule to mark updated nutritional information in the nutrition components tables of food and dietary supplements to help consumer’s eating habits.
Both food and dietary supplement manufacturers that are required to labeling nutrition components tables in their products are subject to obey the all relevant regulations.

In case of nutrition analysis tests during food testing for enter into the US market, require the use of the AOAC method if there is an AOAC verification method.
Through Food nutrition analysis testing by ISO/IEC 17025 recognized IGC LAB by testing methods such as AOAC and BAM, it allows testing and verification of the 14 major nutrients required by the United States and provided sample labels containing nutritional content information.

IGC Food Testing Laboratory is a testing body that has obtained ISO ISO/IEC 17025 accreditation from U.S. IAS.
Based on compliance with the legal requirements related to testing at the product and production sites, the quality policy is to issue an international certified test report that additionally satisfies the quality and ongoing requirements, and the nutrition components, chemical and microbiological test report for enter into the market of domestic companies exporting to the United States.

 FDA Nutritive Components Table Revision form
FDA Nutritive Components Table Revision form
The nutrition analysis table form has been changed newly in 23 years since 1993.
Mandatory application about food manufacturers will begin on July 26, 2018, but companies with annual sales of less than $10 million will be mandatory applied from one year later.
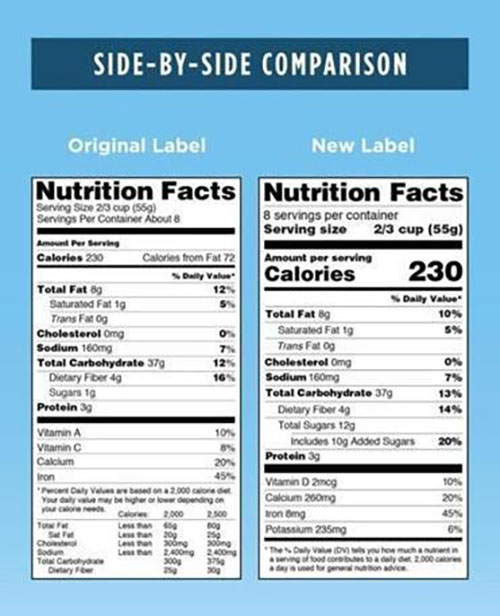
The most notable feature of the newly changed nutrition analysis table form is the large and bold mark of calorie content, serving size, and how many servings it is packed so that consumers can see it well.
Besides, the added sugar content in addition to natural sugars and account for the percentage of this ingredient is recommended daily calorie intake (2,000 calories) are specified separately.
The FDA found it difficult to maintain the daily intake acceptable standards, 2,000 calories or less if the calories from the extra sugar intake exceed 10% of the total calories because results of studies show that about 13% of the calories average daily intake of Americans is from extra sugar.
 Before and after comparison table of the FDA Nutritive Components
Before and after comparison table of the FDA Nutritive Components
On the other hand, the percentage of fat components content displayed highlighting in the label has decreased, active reflecting recent results of studies that show that calories and sugar intake are the main causes of chronic diseases such as obesity and heart disease rather than fat intake itself now. Also, the 'calories from fat' item was excluded and required to the total fat, saturated fat, and trans-fat were marked separately as now.
Moreover, instead of marked the vitamin D and Potassium, which increase the risk of occurring chronic disease in new nutritive components table if lacking, marked the vitamin A and vitamin C content is eliminated. The FDA explained that it is rare for Americans to lack vitamin A and C, while lacking intake of vitamin D and potassium. However, manufacturers can autonomously display vitamin A and C contents, and calcium and iron contents are displayed as they are now.
Nutritive components table regulation applies to almost all packaged foods, but excludes some meat and poultry under the jurisdiction of the Department of Agriculture.
Related Services from IGC
01CE LVD/EMC Certification
02Eurasia Certification
03Product Registration (CPNP, FDA)
04Provide technical support services for testing and certification